The Effect of Hydrotalcite on the Thermal Stabilization of PVC
Polyvinyl chloride (PVC) is one of the most widely used plastics in the world. Thanks to its low cost, flame resistance, mechanical strength, and chemical durability, it is used in pipes, cables, construction materials, packaging, and many other applications. However, the presence of C–Cl bonds in the chain and structural defects such as allylic chlorides and tertiary chlorides make PVC highly unstable when exposed to heat.
PVC begins to undergo dehydrochlorination at relatively low temperatures (around 100 °C). As HCl is released and conjugated polyene chains form, the material undergoes discoloration, a drop in molecular weight, and deterioration of mechanical properties. The released HCl also autocatalyzes further degradation, triggering a “zipper-like” chain-decomposition reaction.

Since industrial processing of PVC typically occurs at 160–210 °C, the use of thermal stabilizers to prevent degradation is unavoidable.
Traditional stabilizers include lead salts, organotin compounds, and metal soaps. Although lead-based stabilizers offered excellent performance, they have been prohibited in many countries because of their severe toxicity. Calcium–zinc (Ca/Zn) systems are safer and appropriate alternatives, but they generally require co-stabilizers to achieve desirable performance.
Among these, hydrotalcite has attracted significant attention as a modern, environmentally friendly, and highly effective stabilizer. Its layered structure and basic nature enable it to absorb and neutralize the HCl released from PVC.
Structure and Properties of Hydrotalcite
Hydrotalcite, with the general chemical formula Mg₆Al₂(OH)₁₆CO₃·4H₂O, is an inorganic anionic nanoclay belonging to the family of layered double hydroxides (LDH). Its structure is composed of positively charged hydroxide layers, separated by interlayer anions and water molecules. In fact, this structure is derived from brucite, Mg(OH)₂, but part of the Mg²⁺ ions are replaced by Al³⁺ ions, and this substitution generates a net positive charge on the layer. This charge must then be balanced by interlayer anions such as carbonate, chloride, or nitrate.


Hydrotalcite structure
Hydrotalcite is capable of effectively absorbing HCl because it contains:
- positively charged layers
- exchangeable interlayer anions
- strong basicity
- a layered structure with high active surface area
This feature is the key reason for its functionality in PVC thermal stabilization.
Mechanism of PVC Thermal Stabilization by Hydrotalcite
The stabilizing effect of hydrotalcite in PVC mainly results from two key processes:
1- HCl Adsorption and Ion Exchange
When PVC decomposes and releases HCl, chloride ions (Cl⁻) enter the interlayer galleries of the LDH and replace the original anions such as carbonate. This ion-exchange process traps HCl and prevents it from returning to the polymer phase.
2- Neutralization of HCl by OH Groups
When a large number of Cl⁻ ions and H⁺ protons accumulate within the hydrotalcite structure, the hydroxyl groups (OH⁻) and the metal cations present in the layers begin to react with HCl. This leads to the neutralization of HCl and the formation of water and metal chlorides (such as MgCl₂ and AlCl₃). The consumption of HCl by hydrotalcite prevents it from diffusing into the polymer and catalyzing the dehydrochlorination process of PVC.
Ultimately, during these reactions, the hydrotalcite structure collapses, and its layers are converted into a mixture of metal chlorides. In general, hydrotalcite acts like an alkaline sponge, capturing and neutralizing HCl at the moment it is formed.
Secondary Effect: Reduced Activation of Labile Chlorides in PVC
Some studies report that the positively charged LDH layers can reduce the electron density on PVC’s chlorine atoms, lowering the probability of their removal. Although this effect is secondary, it helps reduce the rate of yellowing.
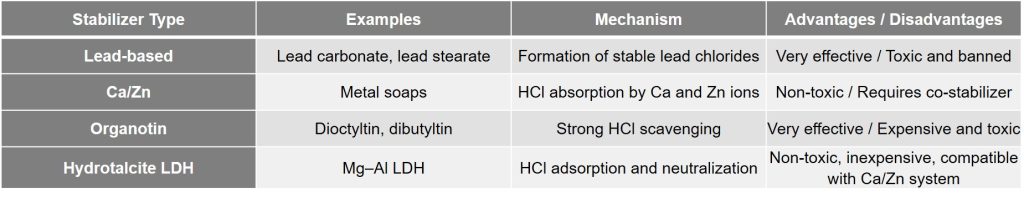
Comparison of Hydrotalcite with Other Stabilizers
The table below summarizes the strengths and weaknesses of the conventional stabilizers used in PVC. Based on this, lead salts—despite their high effectiveness—are no longer desirable options due to the serious health risks they pose (lead poisoning, organ damage). Calcium–zinc stabilizers are now recognized as the industrial standard for rigid PVC. Although these compounds are much safer, they do not act as “intelligently” as hydrotalcite. They typically require organic co-stabilizers (such as β-diketones, epoxides, etc.) to achieve full thermal stability and proper color control. Organotin-based stabilizers (such as dibutyltin dilaurate) provide very strong stabilization and are widely used in flexible PVC, but their toxicity—especially to the nervous system—is a significant drawback.
In contrast, hydrotalcite-based stabilizers are considered completely safe from an environmental standpoint. Hydrotalcite is essentially a non-toxic material (even used as an antacid in pharmaceuticals) and contains no heavy metals in its chemical structure. In fact, adding hydrotalcite to calcium–zinc stabilizer systems can significantly improve their performance. For example, hydrotalcite-based fillers have been shown to enhance the thermal stability and weathering resistance of PVC/Ca–Zn formulations without negatively affecting product color. Therefore, in terms of environmental compatibility, long-term performance, and safety, hydrotalcite offers substantial advantages over many older stabilizer systems.
Performance comparison of common PVC stabilizers
Conclusion
Hydrotalcite and other LDH materials represent a new generation of PVC thermal stabilizers that function based on their layered structure and basic nature. These materials can:
- capture and neutralize HCl on the spot
- suppress the chain-degradation reactions of PVC
- prevent polyene formation and discoloration
- enhance the performance of Ca/Zn systems
- operate without the environmental risks of lead and organotin stabilizers
For these reasons, hydrotalcite is a green, safe, cost-effective, and highly efficient option for the PVC industry, and it is expected to play a growing role in future formulations.
References
- Guo, Y., et al. “Layered Double Hydroxides as Thermal Stabilizers for Poly(vinyl chloride): A Review.” Progress in Polymer Science, 2018.
- Wilkes, C.E., Summers, J.W., Daniels, C.A. PVC Handbook, 2nd ed., Hanser Publishers, 2005.
- European Chemicals Agency (ECHA). “Restrictions on Lead Compounds in Plastics.” Regulatory Report, 2023.
- Gilbert, M. , et al. “Assessment of PVC stabilisation using hydrotalcites – Raman spectroscopy and other techniques“ Polymer Degradation and Stability, 2013.
- Appel , K.E. “Organotin compounds: toxicokinetic aspects“ Drug Metabolism Reviews, 2004.